|
Aramid comes from the words aromatic and amide, this means its molecular structure
contains aromatic groups - carbon atoms in a ring (left) and amide groups - C, H, N and O arranged as below (right)
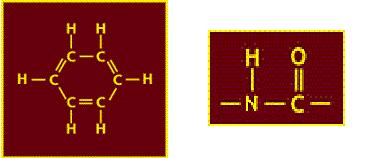
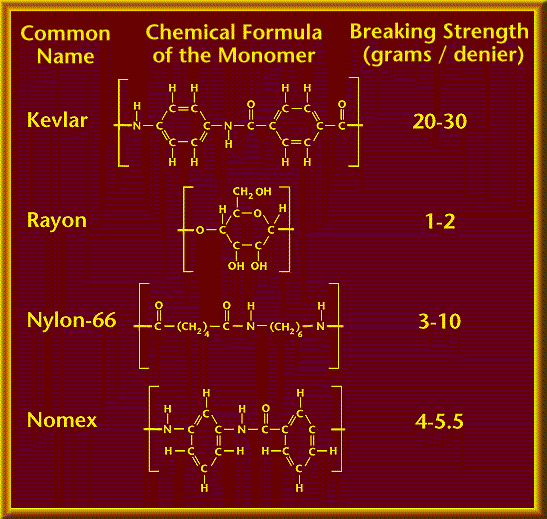
The table below shows that the poly-aromatic amide structure of Kevlar contributes to its strength, note that Nomex (the
second strongest) has two aromatic groups and one amide group. Rayon (the weakest) has no aromatic nor amide groups.
Another factor which contributes to the strength of Kevlar is the way the long-chain molecules are held together. Without
these interatomic forces it would have an amorphous structure which would make it weak and brittle. The interatomic forces
exist because the molecules are polarised, this means that some parts of the chain have a slight positive
charge an others have a slight negative charge. The positive charges on one chain are attracted to the negative charges on
another. Because there are thousands of these charges on one chain and millions of chains in the whole structure, the polymer
is very difficult to break. The diagram below illustrates chemical polarity.
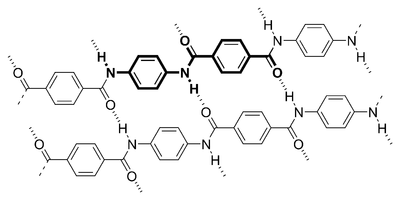
There is an inter-chain attraction between the hydrogen atoms and the oxygen atoms because of their relative electronegativity.
Oxygen and nitrogen have a high electronegativity, this means they have a strong pull on the electron cloud between the atoms
some more electrons come to their side of the bond. This gives them a slight negative charge and the other atom a positive
one. From the diagram we can see that the oxygen atoms have a negative charge because of the oxygen's strong pull on the electrons
in the C=O bond. Conversely, the hydrogen atom has a positive charge because of the adjacent nitrogen atom's pull on the N-H
bond. These charges attract to form the inter-molecular bond.
|

